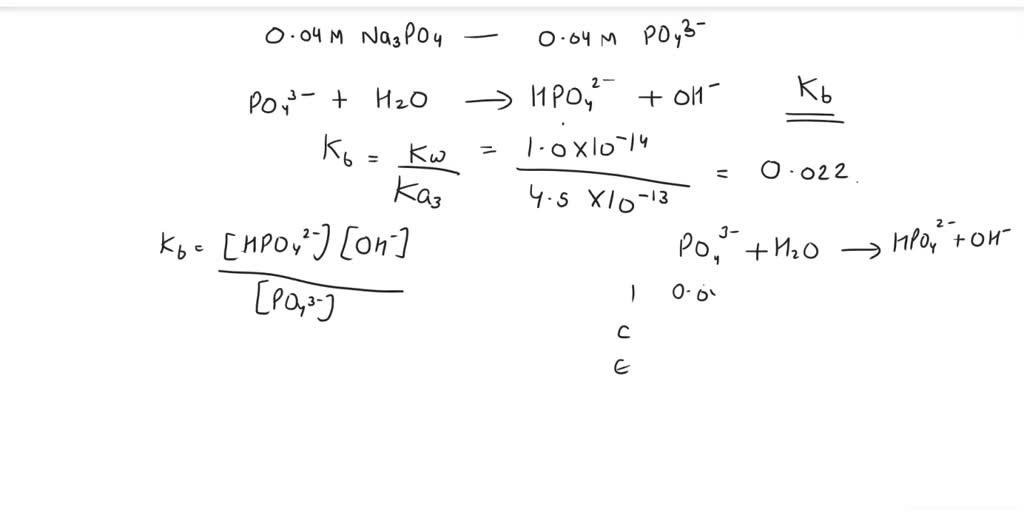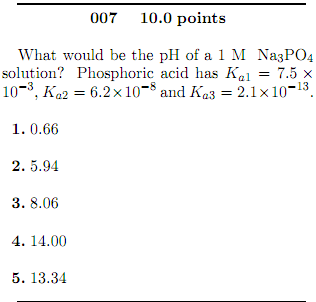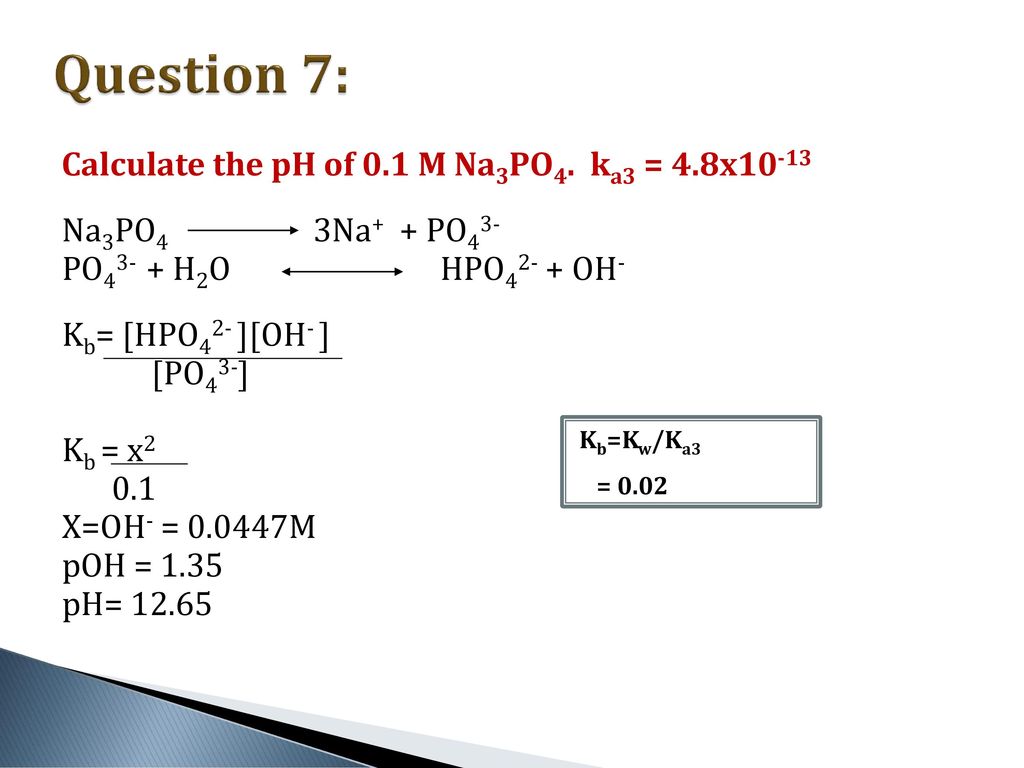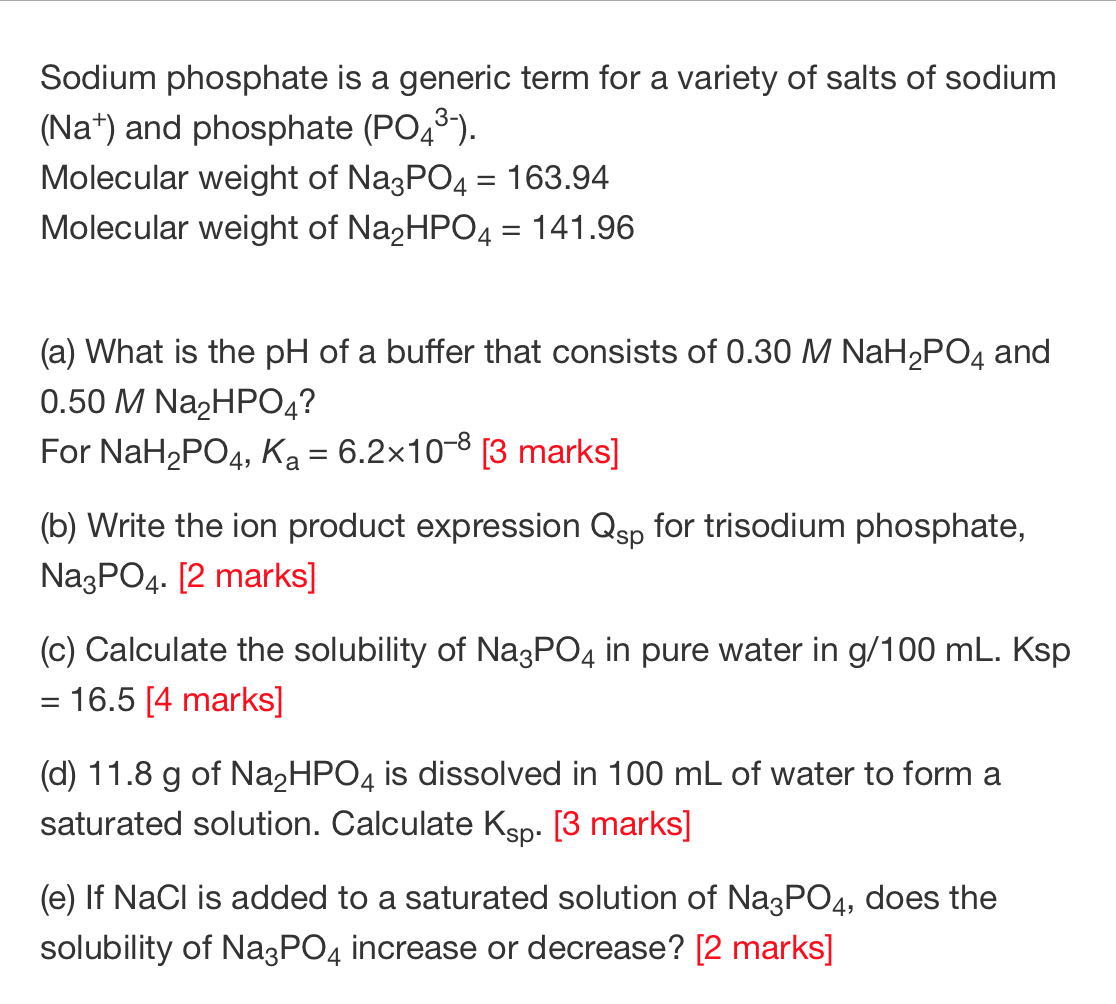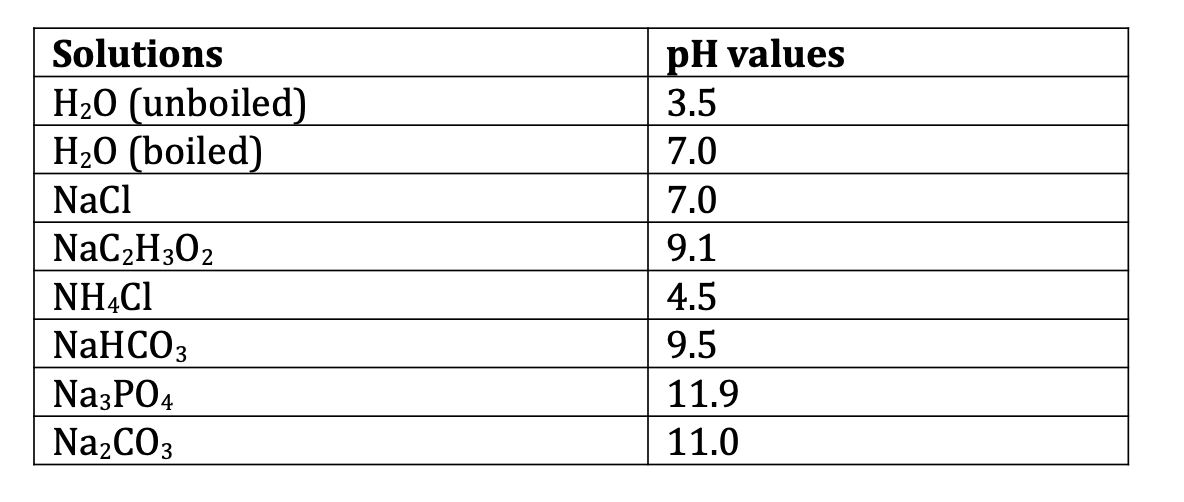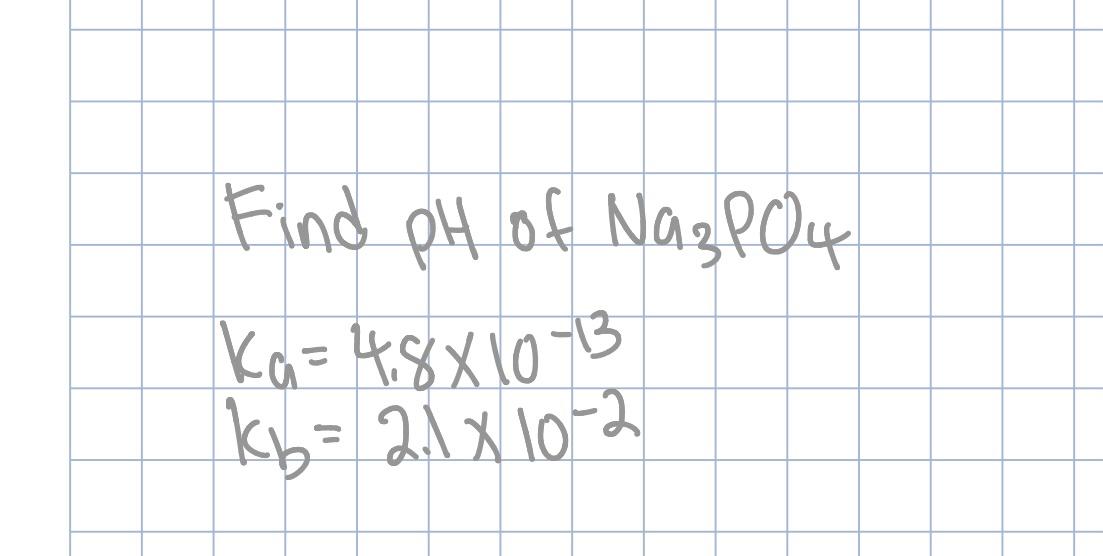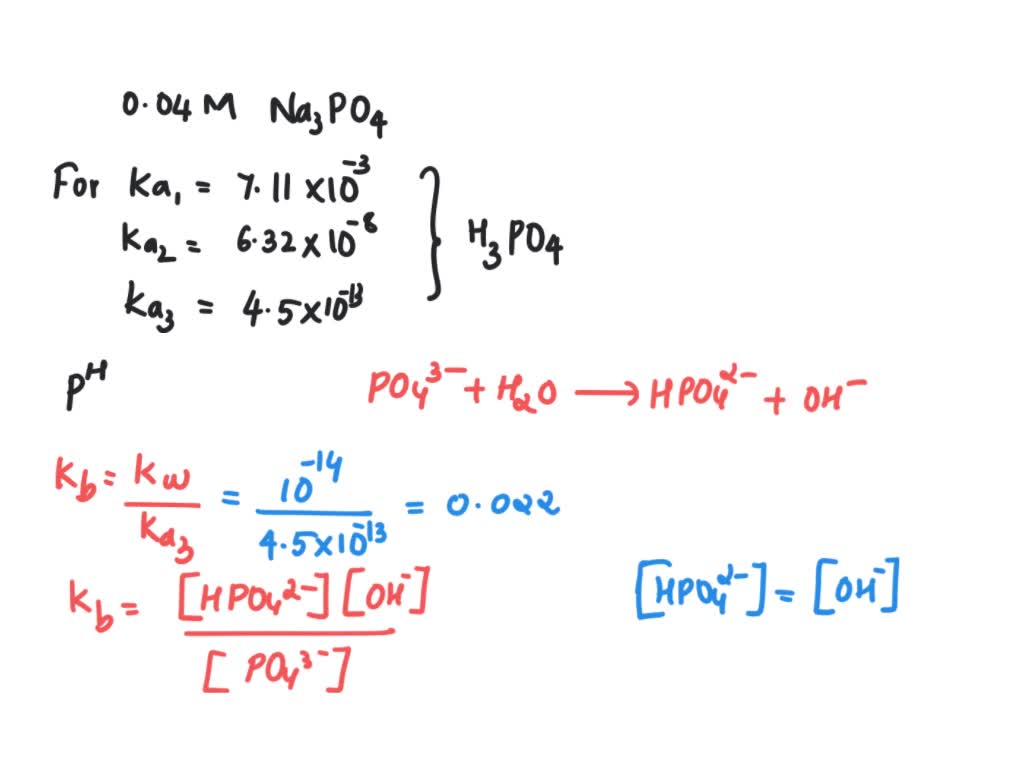
SOLVED: Calculate the pH of a solution that is in 0.04 M Na3PO4. If you made any assumption in a calculation, indicate whether it is valid or not. The magnitude of the

If 2.5 moles each of H3PO4,NaH2PO4,Na2HPO4 and Na3PO4 are mixed together to form an aqueous solution, then the resulting pH is:Given values of Ka are: Ka1 = 10^-3 Ka2 = 10^-7 Ka3 = 10^-13

When 100 mL of 0.1 M KNO3 , 400 mL of 0.2 M HCl and 500 mL of 0.3 M H2SO4 are mixed, then in the resulting solution :