Which one of the following is correct order of second ionisation potential of `Na, Ne Mg` and `Al`? - YouTube

Na Mg Al elementleri için , 1. Birinci iyonlasma enerjileri mg>al>ne 2.Ikinci iyonlasma - Eodev.com
Na, Mg, and Al are 3 elements of the 3rd period in the modern periodic table, having group number 1, 2, and 13 respectively. Which one of these elements has the maximum

SOLVED: Activity Series: Li > K >Ba > Ca> Na >Mg > Al> Mn> Zr > Cr > Fe> Co? Ni> Sn> Pb > H > Cu > Ag > Hg >

Periyodik tabloya göre, Na ,Mg ve Al elementlerinin iyonlaşma enerjilerini büyükten küçüğe sıralayınız. - Eodev.com
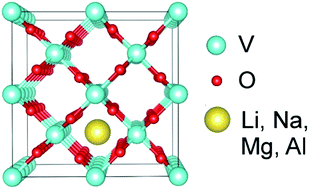
Comparison of Li, Na, Mg and Al-ion insertion in vanadium pentoxides and vanadium dioxides - RSC Advances (RSC Publishing)

In modern periodic table, arrange the third row elements Na, Mg, AI and Si in the increasing order of their atomic size.



![Chemistry] Topic Checklist: Metals and the Reactivity Series Flashcards | Quizlet Chemistry] Topic Checklist: Metals and the Reactivity Series Flashcards | Quizlet](https://o.quizlet.com/yDX1KeN3mt0M8PKXbCGMMQ.png)